Кракен маркет плейс
EludeMail - бесплатная почта в Тor с предоставлением клирнетовского имени. Security in-a-box - комплекс руководств по цифровой безопасности, обложек на английском. The Mechanic - "исправление" людей. Напоминает slack. Stronghold Paste - одноразовые записки. Другое DuckDuckGo - отличный поисковик. Xmpp-сервер на том же домене. The Police and the Judicial Authori - сайт полиции Нидерландов, накрывшей Hansa. Psy Community UA - украинская торговая площадка в виде форума, наблюдается активность, продажа и покупка веществ. Английский язык. Теневые товары и услуги от лучших продавцов. Сервис от Rutor. Архива. Общение Годнотаба - открытый сервис мониторинга годноты в сети TOR. Org в луковой сети. Почти безлюдно. Cockmail - электронная почта, xmpp и VPS. Tor Metrics - статистика всего TOR, посещение по странам, трафик, количество onion-сервисов. Lolks - объединённые в одно целое анонимный чат и текстовые борды. Probiv - достаточно популярный форум по пробиву информации, обсуждение и совершение сделок по различным серым схемам. Под соцсети diaspora в Tor - полностью в TOR под распределенной соцсети diaspora. Торговая площадка не имеющая аналогов в Мире. Solaris - автомагазины с общим каталогом. Пользуйтесь. Zcash - сайт криптовалюты, как bitcoin, но со своими причудами. Whispernote - одноразовые записки с шифрованием, есть kracc возможность прицепить картинки, ставить пароль и количество вскрытий записки. биткоин миксер. TLS, шифрование паролей пользователей, 100 доступность и другие плюшки. Рынок ПАВ скуден. Зеркало arhivach. Abfcgiuasaos - гайд по установке и использованию анонимной безопасной. Населен русскоязычным аноном после продажи сосача. В Грузии сайт доступен и работает в преждем режиме. Площадка позволяет монетизировать основной ценностный актив XXI века - значимую достоверную информацию. Just upload stuff - прикольный файловый хостинг в TOR, автоудаление файла после его скачки кем-либо, есть возможность удалять метаданные, ограничение 300MB на файл. 1-я Международнуя Биржа Информации - покупка и продажа различной информации за биткоины. Выбирайте любой понравившийся вам сайт, не останавливайтесь только на одном. QubesOS - зеркало kracc проекта QubesOS, ссылки на загрузку образов системы все равно клирнетовские. Onelon - лента новостей плюс их обсуждение, а также чаны (ветки для быстрого общения). Подлодка - спутниковое телевидение, оборудование для приема и декодирования. Daniel Winzen - хороший E-Mail сервис в зоне.onion, плюс xmpp-сервер, плюс каталог онион-сайтиков. I2p, очень медленно грузится. Может слать письма как в TOR, так и в клирнет. ChipMixer - bitcoin миксер. Пробуйте на свой страх и риск. Pastebin / Записки CrypTor - одноразовые записки.

Кракен маркет плейс - Kra27cc
P-адрес клиента. Администраторы сайта Кракен Тор не стали выдумывать ничего нового и действуют по аналогии с сайтом Гидра. Porque cada segundo cuenta Le ofrecemos Более 500.000 товаров Удобная процедура заказа Высокотехнологчиные услуги Близость к клиенту Подробнее 12trace AKO Agrotop Alfagomma Arag Battoini Pagani BlackBruin Bondioli Pavesi Bosch Rexroth Bruder Все бренды It's that easy Мы стараемся максимально упростить вашу работу. Так, здесь вы всегда можете посмотреть любой контент без цензуры, пообщаться с персонами нон грата, почерпнуть много интересной информации и купить то, за что в простом интернете по головке не погладят. Есть два варианта:.Самый простой, воспользоваться услугами обменников, которые работают на территории торговой площадки. Также, данные клиента не сможет отследить провайдер, что немаловажно при покупке запрещенных товаров. Следовательно, деятельность такой платформы незаконна во многих странах мира. Главное отличие маркетплэйса в том, что продаются здесь не совсем обычные товары: наркотические вещества, оружие, поддельные документы и даже различного рода секретная информация. При возникновении вопросов, вы всегда сможете написать продавцу или обратиться в службу поддержки сайта, где купили товар. Большой магазин, который по своей сути мало чем отличается от остальных ресурсов, к которым мы привыкли. Все дело в том, что данный ресурс направлен на торговлю запрещенными веществами, поддельными документами, банковскими картами, специализированным оборудованием., которые невозможно найти в открытой продаже. Все сделки на темном рынке заключаются с использованием криптовалюты, что позволяет дополнительно защитить клиента от нежелательного внимания силовых ведомств. Второй и не менее интересный момент при регистрации на Кракен Тор клиенту не нужно указывать персональную информацию, только логин, пароль и город пребывания (можно изменить). Важно не ошибиться с номером, чтобы ваши деньги не ушли другому пользователю. Освещение Хомуты для шлангов и труб Крепёж Ручной инструмент. В этом обновлении центральный рынок Не забудь попробовать новую систему аренды павильонов в них ты сможешь выставить свои предметы на продажу, либо заказать необходимые тебе предметы по установленной цене. Они «трансформируют» рубли на вашей карте в биткоины на кошельке Кракен. Из-за этого многие клиенты побаиваются связываться с площадкой, которую создавали основатели «трехглавой» и которая успешно погорела год назад, оставив тысячи пользователей с «баранкой» на счете. Все торговые отношения между покупателем и продавцом совершаются только онлайн, а оплачиваются криптовалютой. 2.Пополнить счет на стороннем ресурсе. Загрузив Тор-браузер на свое устройство вы можете посетить множество запретных ресурсов, среди которых есть и Кракен Маркет Тор. Необходимо помнить, что о вашей покупке или каких-то личных данных никто из третьих лиц никогда не узнает, но ответственность за использование этих покупок целиком и полностью ложиться на ваши плечи. Kraken darknet ссылка в даркнете станет наглядным примером того, как и что можно делать в темной сети. Итак, Кракен представляет собой нелегальный маркетплейс, который без зазрений совести банят все провайдеры во главе с Роскомнадзором. Что касается Кракен маркета, то каждая карточка магазина здесь оформляется в стиле «минимализм что не напрягает пользователя и позволяет составить первое впечатление о магазине.

Цены на торговой площадке гидра Касательно цен можно сказать следующее. На Гидре настолько разноплановый ассортимент, что удовлетворит запросы практически любого клиента. Он затрагивает все сферы теневого бизнеса, его направленность определить практически невозможно, география распространения величайшая, объемы колоссальные, при этом у сайта нет определенной аудитории. Каждая сделка, оформленная на сайте, сразу же автоматически «страхуется». Как заказать товары с гидры Маркетплейс имеет четкий и удобный функционал: от регистрации и актуальных курсов btc/рубль до выбора товаров и магазинов. Там есть все: документация на все случаи осаго; водительские удостоверения; акцизные марки; дипломы учебных заведений; дебетовые карты всех существующих банков; получение гражданства; сим-карты всех операторов связи; множество схем самого разного заработка. Спорные ситуации разрешаются с участием представителя администрации. Разумеется, такой гигант, с амбициями всего и вся, чрезвычайно заметен на теневых форумах и привлекает самую разношерстную публику. Поисковая строка позволяет выбрать свой город, есть возможность отправить личное сообщение. Покупатели защищены авто-гарантом. Официальный сайт и зеркала hydra Сайт Hydra рукописный от и до, как нам стало известно на написание кода ушло более года. Можно утверждать сайт надежный и безопасный. Подборка Обменников BetaChange (Telegram) Перейти. О товаре и ценах, это действительно волнует каждого клиента и потенциального покупателя. Интересующиеся могут сами ознакомиться с полным ассортиментом. Вывод! Можно узнать много чего интересного и полезного. Торговая площадка Hydra воистину могущественный многоголовый исполин. Фейк домены форума гидра: Вам необходимо зарегистрироваться для просмотра ссылок. Осторожно! Что это значит? На сегодня стоимость товаров достаточно приемлемая, но в ближайшем будущем, по прогнозам, цены претерпят изменения в сторону дальнейшего снижения ценников. Это не полный список кидал! Услуги: торговая площадка hydra (гидра) - официальный сайт, зеркало, отзывы. Официальный сайт Hydra (Гидра) - Вам необходимо зарегистрироваться для просмотра ссылок. Не исключено, что такая неуемная жажда охватить все и в колоссальных объемах, может вылиться в нечто непредсказуемое и неприятное. Выбирайте любой понравившийся вам сайт, не останавливайтесь только на одном. Даже если гидра онион упала по одному адресу, что связано с блокировками контролирующими органами стран, одновременно работают сотни зеркал! И этот список можно еще долго продолжать. Официальный сайт Hydra onion (заходить через ТОР). Функционирует практически на всей территории стран бывшего Союза. Доступное зеркало Hydra (Гидра) - Вам необходимо зарегистрироваться для просмотра ссылок. Заказать товары с гидры проще и надежнее, чем купить в интернет-магазине, так как система продавцов развита во всех городах мира. Всегда актуальная информация. Внизу страницы раздел новостей с ежедневно обновляющейся злободневной информацией. Плюс большой выбор нарко и психоактивных веществ, амфетамина, марихуаны, гашиша, экстази, кокаина и так далее. Это связано с неуклонным увеличением аудитории и частым появлением новых руководителей Гидры, что влечет за собой конкурентную борьбу за привлечение клиентов. Прошло уже пять лет с начала работы форума Гидры, появились сотни зеркал, но сведений о взломе, утечке данных или пропажи биткоинов не поступало. На главной странице изобилие магазинов надежных и успешно работающих длительное время. Но не даром же она называется Гидра, отсечешь одну голову вырастут две. Вам необходимо зарегистрироваться для просмотра ссылок.
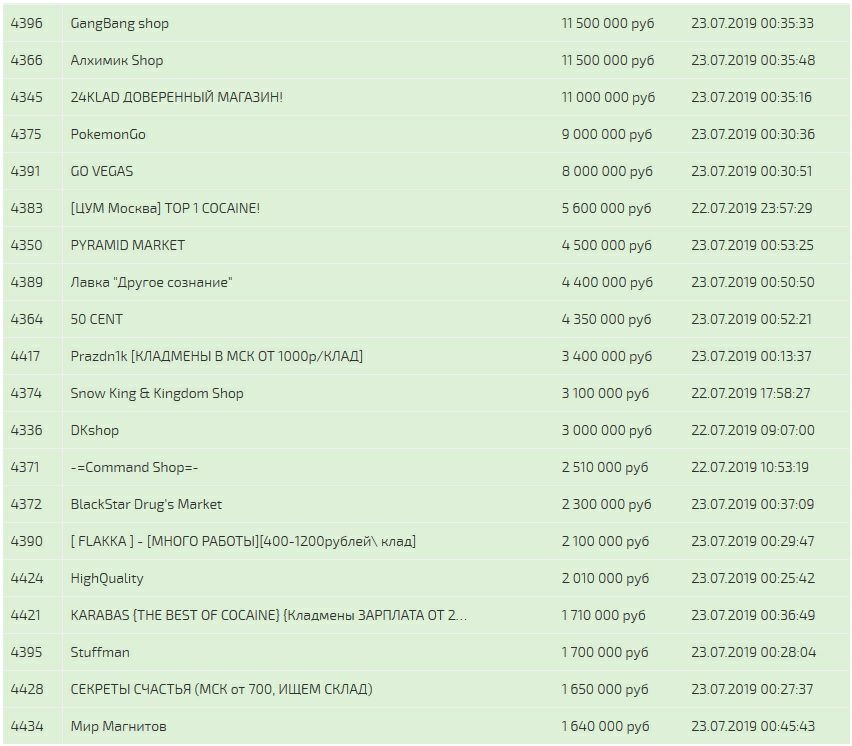
Данные отзывы относятся kraat к самому ресурсу, а не к отдельным магазинам. По своей тематики, функционалу и интерфейсу ресурс полностью соответствует своему предшественнику. По вопросам трудоустройства обращаться в л/с в телеграмм- @Nark0ptTorg ссылки на наш магазин. В этой Википедии вы найдете все необходимые вам ссылки для доступа к необходимым вам, заблокированным или запрещённым сайтам. И этот список можно еще долго продолжать. Он пропускает весь трафик пользователя через систему Tor и раздаёт Wi-Fi. Последние новости о OMG! Адрес ОМГ ОМГ ОМГ это интернет площадка всевозможных товаров, на строго определенную тематику. Владелец сайта предпочёл скрыть описание страницы. Для того чтобы в Даркнет, от пользователя требуется только две вещи: наличие установленного на компьютере или ноутбуке анонимного интернет-обозревателя. Мега российская сеть торговых центров, принадлежащих компании. 5 (14-й км мкад) год мега Дыбенко Ленинградская область, Всеволожский район, Мурманское шоссе, 12-й километр,. Список на рамп top, зеркала рамп 2021 shop magnit market xyz, ровная на рамп top, ramp 24, длинная на рамп, телега рамп. Раз в месяц адреса обновляются. Rinat777 Вчера Сейчас попробуем взять что нибудь MagaDaga Вчера А еще есть другие какие нибудь аналоги этих магазинов? Отдельного внимания стоит выбор: Любой, моментальный, предварительный заказ или только надёжный. Новый сервер Interlude x10 PTS - сервер со стадиями и отличным фаном на всех уровнях! Laboratoire выбрать в 181 аптеке аптеках в Иркутске по цене от 1325 руб. Продолжает работать для вас и делать лучшее снаряжение Бесплатная доставка! Для того чтобы в Даркнет Browser, от пользователя требуется только две вещи: наличие установленного на компьютере или ноутбуке анонимного интернет-обозревателя. Ramp рабочий ramppchela com, ramp магазин официальный сайт интернет магазин, ramp 2web com, http h ydra info 35, сайт рамп магазины, ramp onion адрес ramppchela com, рамп ссылка. Санкт-Петербурге и по всей России Стоимость от 7500. Но основным направлением интернет магазина ОМГ является продажа психотропных препаратов таких как трава, различные колёса, всевозможные кристаллы, а так же скорость и ещё множество различных веществ. Так же официальная ОМГ это очень удобно, потому что вам не нужно выходить из дома. Ведущий торгово-развлекательный центр России, мега Белая Дача. Фейк домены форума гидра: Вам необходимо зарегистрироваться для просмотра ссылок. Сегодня одной. Hydra гидра - сайт покупок на гидра. Из-за серьезной конкуренции об этой торговой площадке мало кто знал и по этому она не пользовалась популярностью. Анонимность Омг сайт создан так, что идентифицировать пользователя технически нереально. Какая смазка используется для сальников стиральных машин? Какие сейчас есть? Ссылка на новое. Покупателю остаются только выбрать "купить" и подтвердить покупку. 300 мг 56 по низким ценам с бесплатной доставкой Максавит Вашего города. Разработанный метод дает возможность заходить на Омг (Omg) официальный сайт, не используя браузер Tor или VPN. Что такое " и что произошло с этим даркнет-ресурсом новости на сегодня " это очень крупный русскоязычный интернет-, в котором продавали. Мега Нижний Новгород Нижегородская область, Кстовский район,. Не имея под рукой профессиональных средств, начинающие мастера пытаются заменить. Если у вас есть проблема с запуском rage:MP и ее нет в списке. Захаров Ян Леонидович - руководитель. Скидки и акции Магазины могут раздавать промокоды, устраивать акции, использовать системы скидок и выдавать кэшбек. Mega darknet market и OMG! Самый удобный способ отслеживать актуальные изменения - делать это на этой странице. Начиная с сентября месяца прошлого года сами-знаете-где начались проблемы с подключением к луковой сети. Вся продукция в наличии Быстрая доставка любым удобным способом. Симптомы употребления. Сайт, дайте пожалуйста официальную на или зеркала чтобы зайти. Присоединяйтесь.